February 24, 2020 | Nora Samaranayake
Long-term HIV Infection Can lead to Inflammatory Abnormalities Such as Cardiovascular Disease
On February 1, the Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) and OncoImmune, Inc. launched a new Phase II clinical trial called CALIBER, which will test whether OncoImmune’s lead product, CD24Fc, can reduce late effects of HIV infection, such as inflammatory abnormalities including cardiovascular disease. This is intended for use in HIV patients who have been receiving the traditional antiretroviral therapies that control, but do not cure, HIV infection.
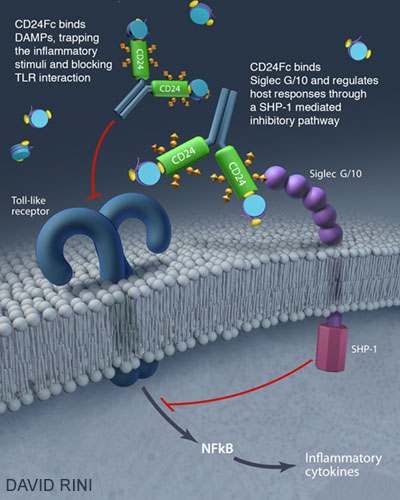
The CD24Fc is an experimental drug made of two parts: one is from a cell surface protein called CD24, and the other is from a constant region of an antibody called Fc. CD24 inhibits inflammation triggered by accidental cell death, while Fc helps to make the drug stay in our body for weeks. Earlier studies in experimental animals and heatlhy volunteers have shown the drug reduces inflammation and “bad” cholesterol, called LDL-C, by stimulating a family of immunoregulatory molecules called Siglec.
The new trial will test the drug for its ability to tone down chronic inflammation and metabolic abnormalities in HIV patients. The trial is supported by a grant from the National Heart, Lung and Blood Institute. Pan Zheng, MD, PhD, Professor of Surgery at the Institute Human Virology, University of Maryland School of Medicine and Chief Medical Officer at OncoImmune, and Shyam Kottilil, MBBS, PhD, Professor of Medicine and Director of the Clinical Care and Research Division at the Institute of Human Virology, University of Maryland School of Medicine, are the co-principal investigators of the grant.
HIV affects an estimated 36.7 million people worldwide. In the United States, an estimated 1.1 million people are infected. As access to antiretroviral treatment (ART) has increased, the life expectancy of people infected with HIV has increased. However, among people living with HIV, increased cardiovascular disease and other metabolic disorders contributes to an increased risk of morbidity and mortality.
“Since chronic inflammation is the major underlying cause of the increased risk, a critical challenge is how to control chronic inflammation in HIV patients,” said Dr. Kottilil.
CALIBER is a Phase II, randomized, double-blind, placebo-controlled clinical trial. A cohort of 64 subjects with HIV on antiretroviral therapy will be randomized in a 1:1 fashion to be administered with CD24Fc or placebo during a 4-week window, followed by a 24-week follow-up period to assess safety and efficacy in normalizing lipid profiles, reducing inflammation in the cardiovascular system and liver, as well as reducing immune abnormalities found in HIV patients.
“OncoImmune’s CD24Fc dampens inflammation in HIV patients,” said Dr. Zheng. “Therefore, it holds the promise to reduce many health risks imposed by HIV infection, including heart and liver diseases.”
“This trial is a terrific example of the collaboration between IHV and OncoImmune,” said Robert Gallo, MD, the Homer & Martha Gudelsky Distinguished Professor in Medicine and Co-Founder and Director of the Institute of Human Virology, University of Maryland School of Medicine. “It leverages the intellectual resources as well as the strong clinical infrastructure at IHV, and the expertise of OncoImmune as a leader in fortifying the CD24-Siglec checkpoint of innate immunity. This checkpoint was originally discovered by IHV faculty and OncoImmune co-founders, Drs. Yang Liu and Pan Zheng.”
About the Institute of Human Virology
Formed in 1996 as a partnership between the State of Maryland, the City of Baltimore, the University System of Maryland and the University of Maryland Medical System, IHV is an institute of the University of Maryland School of Medicine and is home to some of the most globally-recognized and world-renowned experts in all of virology. The IHV combines the disciplines of basic research, epidemiology and clinical research in a concerted effort to speed the discovery of diagnostics and therapeutics for a wide variety of chronic and deadly viral and immune disorders - most notably, HIV the virus that causes AIDS. For more information, www.ihv.org and follow us on Twitter @IHVmaryland.
About OncoImmune, Inc.
OncoImmune (www.oncoimmune.com) is a privately-held, clinical-stage biopharmaceutical company that is actively engaged in the discovery and development of novel immunotherapies for cancer, inflammation and autoimmune diseases. OncoImmune is based in Rockville, Maryland. OncoImmune’s lead product, CD24Fc, is a novel therapeutic that regulates host inflammatory response to tissue injuries and which has broad implications in the pathogenesis of cancer, autoimmune disease, metabolic syndrome and graft-versus-host disease (GvHD). CD24Fc has completed a Phase IIa trial for the prophylactic treatment of acute Graft versus Host Disease (aGvHD) in leukemia patients undergoing hematopoietic stem cell transplantation (HSCT) and resulted in a significant improvement in 180 Day Grade III-IV acute GVHD Free Survival, the Phase III primary endpoint. CD24Fc prophylaxis also resulted in reduced relapse and, compared to match controls, CD24Fc demonstrated improvement in Overall Survival, Non-Relapse Mortality and Relapse-Free Survival. A dose-dependent reduction in severe (Grade > 3) mucositis was also observed. A 20 patient open label dose expansion cohort at the recommended clinical dose is fully enrolled and the drug continues to perform very well. In addition to the CALIBER trial, a Phase III study for the prevention of aGVHD is anticipated to start in early 2020.
Contact
Institute of Human Virology
Jennifer Gonzales
Public Relations & Communications Manager
jennifer.gonzales@ihv.umaryland.edu
Institute of Human Virology
Nora Samaranayake
nsamaranayake@ihv.umaryland.edu
410-706-8614
OncoImmune, Inc.
Martin Devenport
mdevenport@oncoimmune.com
Related stories

Wednesday, December 01, 2021
$6.5M Grant Awarded to Develop Treatment for Alcoholic Liver Disease-Associated Kidney Dysfunction
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) and MitoPower LLC (“MitoPower”) were awarded an SBIR (Small Business Innovation Research) grant of up to $6.5 million over five years from the National Institute on Alcohol Abuse and Alcoholism. The funds will support the development of MitoPower’s lead compound, MP-04, for the treatment of kidney dysfunction due to alcoholic liver disease, a condition known as alcoholic liver disease-associated hepatorenal syndrome (HRS). The IHV, a Center of Excellence member of the Global Virus Network (GVN), will conduct first-in-human single and multiple ascending dose studies to test the safety of the compound, followed by a Phase 1b study in patients.

Tuesday, December 15, 2020
UMSOM Institute of Human Virology’s Shyam Kottilil, MBBS, PhD Receives Top Award from National Physician’s Group
Shyam Kottilil, MBBS, PhD, professor of medicine at the University of Maryland School of Medicine (UMSOM), and Director of UMSOM’s Institute of Human Virology (IHV) Division of Clinical Care and Research, has been awarded Mastership in the American College of Physicians (ACP), the national organization of internists. Dr. Kottilil is also Chief of the Division of Infectious Diseases in the UMSOM Department of Medicine and is a scientific advisory member of the Global Virus Network (GVN).

Friday, December 13, 2019
Institute of Human Virology Scientists Propose New Theory for Cancer Immunotherapy
Scientists from the Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) this week proposed a new theory, based on the findings of three previously published studies, to improve both the efficacy and safety of immunotherapy drugs. The theory calls for a new design for these drugs that would prevent side effects like life-threatening immune system reactions and enable a higher dose of the drug to be administered.

Tuesday, December 11, 2018
Institute of Human Virology's Shyam Kottilil to Receive National Award from American College of Physicians
The American College of Physicians announced that Shyam Kottilil, MBBS, PhD, FACP, Professor of Medicine and Director of the Division of Clinical Care and Research at the Institute of Human Virology (IHV) of the University of Maryland School of Medicine (UMSOM) and Chief of the Division of Infectious Diseases, was awarded the American College of Physicians (ACP) Richard and Hinda Rosenthal Award #1 from the Rosenthal Family Foundation.

Thursday, May 03, 2018
UM School of Medicine's Institute of Human Virology Makes Key Appointments to Clinical Care and Research Leadership Positions
Institute Names Dr. Shyam Kottilil and Dr. Anthony Amoroso to replace Dr. Robert Redfield, now Director at CDC.

Wednesday, February 28, 2018
Institute of Human Virology Adds Team of Top Cancer Immunotherapy Experts
Robert C. Gallo, MD, the Homer & Martha Gudelsky Distinguished Professor in Medicine at the UMSOM and Co-Founder & Director.of the UMSOM’s IHV, announced today that a team of leading scientists in the growing area of immune therapeutics for cancer treatment and organ transplantation, led by internationally-recognized cancer researchers Yang Liu, PhD, and Pan Zheng, MD, PhD will be joining the IHV with academic appointments in the UMSOM Department of Surgery.